In the in vitro diagnostics (IVD) industry, every plastic component is more than a molded part — it is a precision-engineered element that directly influences assay accuracy and patient outcomes. From microfluidic cartridges to reagent chambers and sealing components, a deviation of just a few microns can lead to fluid imbalance, inconsistent test results, or costly recalls.
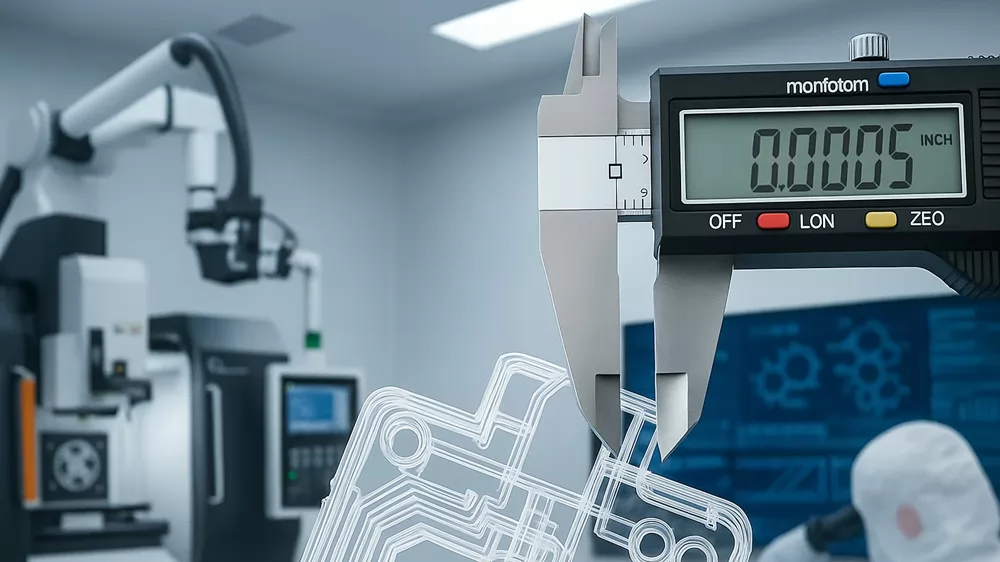
That’s why IVD molding demands extreme accuracy, stability, and repeatability. As a leading IVD molding manufacturer, TPI specializes in high-precision medical plastic injection molding for diagnostic and drug delivery applications — achieving up to 0.0005 inch tolerance through advanced tooling, process control, and validation systems.
How Precision Shapes Diagnostic Reliability
1. Assay Performance Depends on Dimensional Control
Microfluidic channels, reaction wells, sealing membranes, and plungers all rely on perfect geometry. Even a 0.002 inch variance in wall thickness or alignment can alter reagent flow rates, pressure distribution, and assay timing — directly impacting diagnostic accuracy and reproducibility.
2. Assembly Fit and Tolerance Stack Management
Most IVD devices are assemblies of multiple molded parts — cartridges, lids, seals, and inserts. Precision molding minimizes tolerance stack-up and ensures seamless assembly, preventing leaks, warpage, and stress during device operation.
3. Regulatory and Safety Requirements
Every diagnostic product must comply with FDA, CE, and ISO 13485 standards. Maintaining ultra-tight tolerances reduces out-of-spec risks, improves traceability, and supports full process validation — all critical for medical compliance and patient safety.
How to Achieve 0.0005 inch Accuracy in IVD Injection Molding
Reaching this level of micro-precision requires excellence across tooling, materials, process control, and quality systems.
1. Ultra-Precision Tooling & Mold Design
• In-house 5-axis machining and fine polishing deliver mirror-quality mold surfaces.
• Balanced hot-runner / valve-gate systems ensure uniform fill and eliminate shear or distortion.
• Optimized cooling and thermal control prevent warpage and dimensional drift.
• Simulation-driven compensation accounts for polymer shrinkage and flow behavior.
2. Medical-Grade Material Selection
• Use polymers with stable shrinkage coefficients and low anisotropy.
• Consider hygroscopic behavior, moisture absorption, and long-term dimensional stability.
• Adjust mold geometry based on empirical shrinkage data and part geometry.
3. Scientific Process Control
• Apply Scientific Injection Molding (SIM) or decoupled molding to isolate each process phase.
• Maintain strict control of melt temperature, pressure, and cooling time.
• Utilize pressure and temperature sensors for in-line monitoring.
• Implement SPC (Statistical Process Control) to detect and correct deviations early.
4. Precision Metrology & Quality Assurance
• Use high-accuracy CMMs (contact and optical) for dimensional verification.
• Establish robust measurement protocols: environmental control, multi-point sampling, and fixture repeatability.
• Maintain full traceability with part-level data and nonconformance alerts.
• Apply 100% in-line inspection for critical components when feasible.
5. Cleanroom Manufacturing & Validation
• Operate within ISO Class 8 cleanrooms under GMP standards.
• Conduct full IQ / OQ / PQ process validation and change control.
• Maintain complete documentation for regulatory audits and client review.
Why Choose TPI for IVD Injection Molding

• Purpose-Built for Diagnostics: 100% focus on IVD and medical applications, not general consumer parts.
• Unmatched Precision: Consistent 0.0005 inch accuracy, cycle after cycle.
• Regulatory Readiness: ISO 13485 certification, cleanroom production, validation support.
• Global Presence: Dual manufacturing sites in China and the U.S., ensuring supply security and flexibility.
• Partnership Approach: From DFM to mass production, we collaborate to optimize cost, reliability, and scalability.
Conclusion
As the IVD market continues to expand rapidly, precision molding will define the future of diagnostic innovation.
Choose a partner equipped with 0.0005 inchaccuracy, ISO 13485 certification, and proven IVD molding expertise.
Ready to start your next diagnostic project? Contact our team today to discuss your IVD injection molding requirements and accelerate your path to commercialization.